CONTENTS
PART: 1 The Impact of Microorganisms in Pharmaceutical and Medical Device Manufacture
The Essentials of Pharmaceutical Microbiology
by Tim Sandle and Madhu Raju Saghee
Introduction
History of Microbiology
Fundamental Characteristics of Microorganisms
Bacteria
Fungi
Protozoa
Algae
Viruses
Microbial Taxonomy
Microbial Growth
Microbiological Culture Media
Identification and Characterization of Microorganisms
Types of Bacteria
Overview of Pharmaceutical Microbiology
The Scope of Pharmaceutical Microbiology
Product Related Testing Regimes
Starting Materials
In-Process Samples / Intermediate Product
Final Product Formulations
Finished Product
Testing of Utilities
Environmental Monitoring
The Application of Pharmaceutical Microbiology
Counting
Sampling
Microorganisms Detected From Pharmaceutical Manufacturing Environments
Contamination Control
Other Microbiology Laboratory Tests
Microbial Identification
Water Activity
Disinfectant Efficacy Testing
Antimicrobial Susceptibility Testing
Microbial Immersion Studies
Cleaning Validation Studies
Investigation of Out of Limits and Out of Trend Results
Microorganisms Used In Pharmaceutical Microbiology Laboratories
Other Activities
Conclusion
References
Relevance of Microorganisms in Pharmaceutical Processing
by Ossama M. El-Tayeb
Introduction
Historical Review of the Involvement of Microbiology with Pharmaceutical Practice
Implications of aseptic surgery, personal and public hygiene
Implications of Food Microbiology
Implications of the introduction of parenteral therapy: {earliest procedures for
sterilization and preservation}
Early tests for sterility: influence of diagnostic medical microbiology
Early pharmacopoeial guidance on sterilization and sterility testing
Historical Review of Science-Based Pharmaceutical Sterilization and Microbiological Quality Control
Re-definition of the pathogenicity of microorganisms
Contributions of the study of microbial injury and death
Contributions of the food processing technologies: the over-kill approach to sterilization
Contributions of the aerospace and electronics industries.
Hospital sterilization and Central Sterile Supply vs. on site sterilization
Impacts of material sciences and development of cold sterilization methods
Filtration as a breakthrough and the bio-burden approach to sterilization.
Impact of statistical fallacies in sterility testing and population death kinetics.
The Broader Picture of Microorganisms and Pharmaceutical Manufacturing: Challenges, Solutions and Pharmacopoeial Guidance
Microorganisms and Ophthalmic Dosage Forms
Microorganisms and Oral Dosage Forms
Microorganisms and Topical Dosage Forms
Microbiological Challenges, Solutions and Pharmacopoeial Requirements
Current Pharmacopoeial Guidance
The Fundamental Concepts
Sampling for Microbial Quality Control and the Microbiologically Homogenous
Batch
Quality Assurance as an Essential Element in Microbial Quality
The Roles of the Microbial Quality Control Laboratory
Current Pharmacopoeial Guidance {on sterilization, sterility testing and microbial
quality of non-sterile dosage forms}
The Future
Will all Pharmaceuticals be Aseptically Processed?
The future of the Microbiological Quality Control Tests
References
Microbial Contamination and Spoilage
by David G. Allison
Introduction
Sources of Microbial Contaminants
Raw Materials
Materials of Natural Origin
Microorganisms from Plant Material
Microorganisms from Animal Sources
Microorganisms from Mineral-Derived Materials
Synthetic Raw Materials
Water
Types of Water
Disinfection of Water
Microbial Contamination from the Manufacturing Environment
Air supply
Equipment and facilities
Personnel
Users / consumers
Factors affecting Microbial Spoilage of Pharmaceutical Products
Preparation and Storage
Nature of the Contaminant Inoculum
Moisture Content
Nutritional Factors
pH and Redox
Consequences of Microbial Growth
Microbiological Control of Raw Materials
Summary
References
Microbiological Considerations in Medical Device Industry
by Martell Winters
Introduction
The Medical Device Microbiology Life Cycle
Inherent and Accumulated Microbial Contamination
Product Design Microbiology
Raw Material and Component Microbiology
Shipping and Storage of Raw Materials and Components
Environmental Microbiology
Product Assembly Microbiology
Cleaning of Products
Packaging Process Microbiology
Sterilization Process Microbiology
Product Use Microbiology
Medical Device Microbiological Contamination
Controlling Product Bioburden
Aseptic Technique
No Magic Answers for Bioburden Control
Use of Bioburden Testing to Improve Manufacturing Microbiology
Use of Sterility Testing to Improve Manufacturing Microbiology
Microorganisms of Concern
Microbiological Challenges for Products with Tissue Components
References
PART: 2 Aspects of Microbiological Quality Control
Selection of Microbiological Culture Media and Testing Regimes
by Tim Sandle
Introduction
Types of Culture Media
Media Manufacture
Quality Control of Culture Media Supplier
When to Perform Quality Control?
Establishing a Nutritive Properties Testing System
Micro-organisms: Typed Cultures
Micro-organisms: Environmental Isolates or ‘Wildtypes’
Micro-organisms: The Microbial Challenge
Micro-organisms for the Testing of Selective Media or Differential Media
Test Method
Test Regime
Sterility Test of Media
Expiry Time Assessment of Culture Media
New Lots of Media
Media Release and Quarantine
Conclusion
References
Microbial Identification
by Ziva Abraham
Importance of Microbial Identification
Isolation of Microorganisms
Staining Methods
Gram Staining
Spore Stain
Fluorescent Stains
India Ink (Colloidal Carbon) Stain
Acid-Fast (Ziehl Neelsen) Stain
Lactophenol Cotton Blue Stain
Microscopy
Stereo Microscope
Bright Field Microscopy
Dark Field Viewing
Phase Contrast Microscopy
Differential Interference Contrast (DIC) Microscopy
Florescent Microscopy
Pre-Differentiation or Confirmation
Taxonomy, Classification, Identification and Nomenclature
Phenotypic Microbial Identification Systems
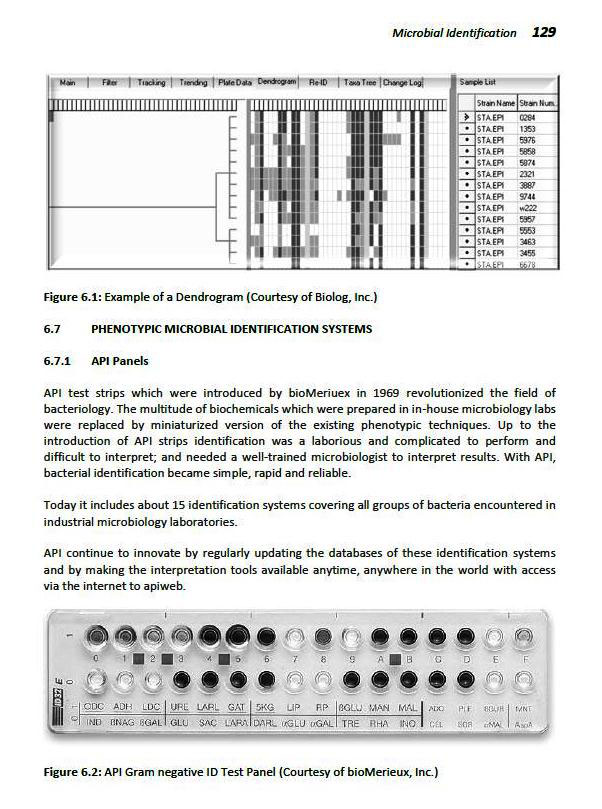
API Panels
Automation in Microbial Identification
Vitek® Microbial Identification Systems
BiologTM System
MIDI Sherlock® Microbial Identification System using Fatty Acid Methyl Ester
(FAME) Analysis
Genotypic Systems
Polymerase Chain Reaction (PCR)
DiversiLab® Strain Typing
Microarrays in Microbial Identification
Fungal Identification
Conclusion
Acknowledgements
References
Microbial Examination of Non-sterile Products
by Jaymie Tomes and Florence Wu
Introduction
The Limitations and Values of Microbial Enumeration Tests
Product Sampling for Microbial Examination
Sampling Schemes
Sample Sizes
Sample Preparation
Media Growth Promotion Test
Method Suitability Test
Enumeration Methods
Media and Incubation
Colony Counting and Interpretation of the Results
Tests for Specified Microorganisms
Staphylococcus aureus
Pseudomonas aeruginosa
Escherichia coli
Salmonella
Clostridium
Candida albicans
Bile Tolerant Gram Negative
Setting Microbial Limits for Non-Sterile Pharmaceuticals and Raw Materials
Conclusions
References
Practical Approaches to Sterility Testing
by Tim Sandle
Introduction
The Sterility Test
Sterility Test Method Validation
Validation of Culture Media
Sterility Test Validation
Dealing with ‘Difficult’ Products
Membrane Filtration
Type of Membrane Filter
Type and Number of Rinse Solutions
Direct Inoculation
Type of Neutralising Agent
Dilution
Turbid Samples
Antibiotics
Oily Samples
Anti-cancer Treatments
Implants
Sterile Aerosols
Cell Lines
Fibrin Sealant
Questions relating to validation that are not directly answered by the Pharmacopoeias
The Stasis Test
Training Programme
Validation Documentation
Validation Protocol
Validation Report
Summary
References
Microbial Aspects in Cleaning Validation
by Andrew Walsh
Introduction
Regulatory Aspects of Cleaning
API Manufacturing
Finished Pharmaceuticals
Cleaning Processes
Cleaning Agents
Cleaning Procedures
Manual Cleaning
Clean-Out of-Place (COP)
Clean-in-Place (CIP)
Solid Dosage Forms
Setting Microbial Acceptance Criteria for Solid Dosage Forms
Semi-solid Dosage Forms
Setting Microbial Acceptance Criteria for Semi-solid Dosage Forms
Hold Time Studies
Microbial Testing in Cleaning Validation
Conclusion
Acknowledgements
References
Validation of Microbiological Methods
by Sandy Rubio
Introduction
Scope
Why is Bioburden Testing Important?
Strategy for Validation
Protocol Considerations
Description of Product
Manufacturing Process
Challenge Microorganisms
Current Validation Status
Regulatory and Guidance Documents
General Protocol Considerations
Bacteriostasis/Fungistasis Evaluation
Acceptance Criteria
Method Development
Sample Hold Time Determination
Maintaining Status of Validated Methods
Case Study
Background
Review Findings
Conclusion
References
Selection and Validation of Disinfectants
by Paul Viña, Sandy Rubio and Tim Sandle
Introduction
Types of Disinfectants
Non-Oxidizing Disinfectants
Alcohols
Aldehydes
Amphoterics
Acid Anionics
Biguanides
Phenolics
Quaternary Ammonium Compounds (QACs)
Oxidizing Disinfectants
Halogens
Oxidizing Agents
Selection of Disinfectants
Number, Type, and Location of Microorganisms
Number
Type of Microorganism and Disinfectant Resistance
Location of Microorganisms
Concentration
Time
Temperature and pH
Interfering Substances
Validation of Disinfectants
Test Methods
Basic Suspension Test
Bactericidal Suspension Test and Fungicidal Suspension Test
Surface Test
Test Variables
Challenge Microorganism Selection and Inoculation of Surface Materials
Selection of Test Surfaces
Disinfectant Test Concentration and Preparation
Establishing the Disinfectant Expiration Date
Evaluating Interference from Organic Material
Application of Disinfectant to Surface Coupons
Contact Time
Selection of Neutralizing Agents
Continued Evaluation of Disinfectant Effectiveness
Conclusion
References
Further Reading
Auditing a QC Microbiology Laboratory
by Andy Martin
Introduction
What is an Audit and Why Should They be Performed
General Principles of the Audit Process
Auditing the QC Microbiology Laboratory, What to Look For
Strategy for Microbiological Control
Sample Receipt
Product Sample Testing
Bacterial Endotoxin Testing
Media Control and Testing
Incubator, Refrigerator and Freezer Control and Monitoring
Culture Collections
Water Testing and Water System Monitoring
Environmental Monitoring
Isolate Identification, Gram stain techniques and ID system validation
Autoclave control
Antibiotic assays and Preservative testing
Sterilization, Cleaning and disinfection validation efficacy
Microbiological Training of Laboratory and Production Staff
Validation of Holding Times
Atypical Results and Out Of Specification Procedures
Auditing Techniques to Get the Most from your Audit
Reporting the Audit
Final Thoughts
References
Quality Assurance in a Microbiology Laboratory
by Christophe Barcella
Introduction
Quality Management, QA and QC Principles
Quality Management
Quality System
Quality Assurance
Quality Control
Laboratory Quality Manual
Laboratory Staff Qualifications and Training
Training Policy
Job Description
Curriculum Vitae
Staff Training Records
Laboratory Environment
General
Air Ventilation
Lab Sanitation and Environment Monitoring
Environmental Hygiene and Health
Protective Equipment
Standard Operating Procedures, Methods and Protocols
Development, Review and Approval of SOPs
New Method Development, Validation and Amendment
Quality Attributes for a New Method
Laboratory Notebooks, Log Books and Records
Minimum Acceptable Procedures for Laboratory Notebooks
Review of Test Results
Electronic Records
Information Technology and Computer Systems Validation
Definition of Hardware and Software
Computer Security and Backups
Computer Systems Validation (CSV)
Archiving Requirements
Archiving Physical Facilities
Archiving Personnel
Archiving Electronic Records
Format of Archived Materials
Laboratory Test Samples and Preserved Wet Materials
Duration of the Archive
Reference Standards and Samples
Reference Standards for Microbiological Testing
Reference Standards Storage, Handling and Labeling
Standard References Records
Microbiological Reference Strains
Management of Samples
Solutions, Reagents and Culture Media
Reagent General Specifications
Reagent Tracking
Reagent Solutions
Quality Control of Culture Media
Instruments and Equipment
Equipment Installation and Qualification
Equipment Operations
Equipment Calibration and Maintenance
Glassware and Volumetric Micropipettes
Glassware Grades
Micropipettors
Laboratory QA Assessments/Audits & External Quality Assurance
QA Audit Methodology
External Quality Assessment (EQA)
Internal Quality Assessment (IQA)
Conclusion
References
Part: 3 Measuring and Testing for Microorganisms
Environmental Monitoring
by Tim Sandle
Introduction
Cleanrooms
Isolators
Cleanroom Classification
Physical Parameters
Air-patterns and Air-Movement
Airflows
Air Changes
Clean-up Times (Recovery Rate Test)
Positive Pressure
HEPA Filters
Temperature, Humidity, Lighting and Room Design
Microbiological Environmental Monitoring
Viable Monitoring Methods
Swabs
Contact Plates
Active Air Monitoring
Types of Active Air-Sampler
Settle Plates
Non-viable Monitoring Methods
Establishing an Environmental Monitoring Programme
Sampling Plans
Sample Frequencies
Sample Limits
Personnel
Aseptic Technique
Other Cleanroom Disciplines
Clothing
Cleaning
Summary
References
Microbial Content Testing of Pharmaceutical and Biotechnologically Derived Products
by Dilip Ashtekar and Tim Sandle
Introduction
General Methods for the Quantitative Determination of Viable Count
Membrane Filtration
Pour Plate
Surface Spread plate
Most Probable Number
Evaluation of Results by Various Enumeration Methods
Points to Consider for Counting CFU
Rounding and Averaging
Significant Figures
Countable Range
Statistical Errors from Low Counts
Dilutions
Replicate Plate Counts
Consideration of Performing of Replicate Samples in Place of Testing One Large Sample
Selection of Method for the Enumeration of Microbial Count
Estimating Number of Micro-Organisms in Suspension for Use in Bioburden
Validation or Suitability Test for MLT
Bioburden Testing
Bioburden Test Method Validation
Validation Requirements
Microbial Limit test (MLT)
A1: The TAMC Test
Reporting of Enumeration Test Results
Spreaders
Suitability of the Enumeration Method in the Presence of Product
Test for Specified Organisms
Applied Aspects of Bioburden Testing
Method Limitations
Sample Expiry
Incubation Parameters
Influencing Factors
Staff Proficiency Training
Plate Counting
Pour Plates
Conclusions
References
Bacterial Endotoxins Test
by Masakazu Tsuchiya
Introduction
History of Bacterial Endotoxins Test
Endotoxin
Structure and Biological Activity of Endotoxin
Stability of Endotoxin
Standard Endotoxin
Variability of Potency of Daily RSE Dilutions
Limulus Amebocyte Lysate
Principle
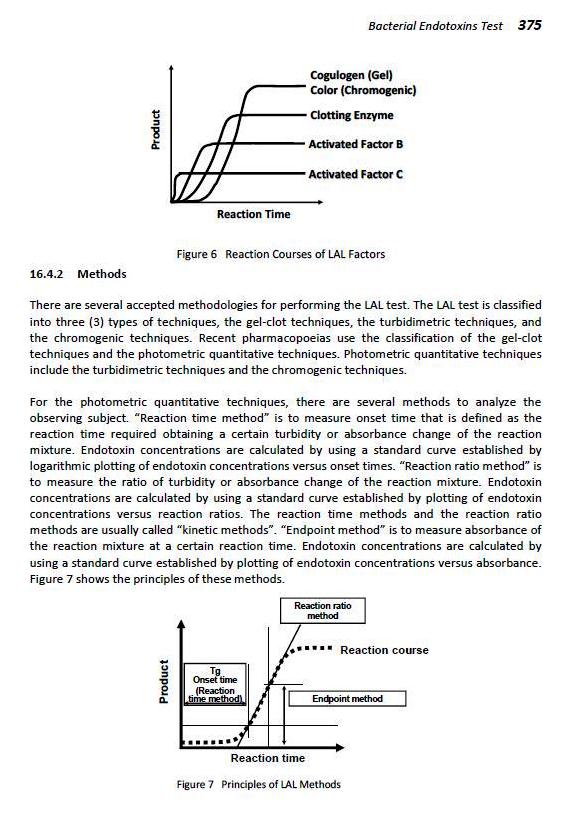
Methods
Reactivity of LAL
Pyrogenicity and LAL Test
Bacterial Endotoxins Test in Pharmacopoeias
Validation of Bacterial Endotoxins Test
Practical Suggestions for the Bacterial Endotoxins Test
Uncertainty of Bacterial Endotoxins Test
Interfering Factors
Contamination
Removal of Endotoxin/β-Glucan
Robust LAL Methods Using an Archived Standard Curve
Conclusion
References
Acknowledgment
Appendix A (Example of Standard Operating Procedure for Gel-Clot Method)
Antimicrobial Effectiveness Testing
by Scott V. W. Sutton
Introduction
The Purpose of the AET
What the Test Means
USP
Pharm Eur
Development of the USP Test
USPXVIII - The Original Test
USP XIX - Refinements
USP XX, XXI and XXII – Little Activity
USP 23, 24 and 25 - Reducing Variability
USP 23, 24 and 25 - Harmonization?
Demonstration of Method Suitability
Importance of Preservative Neutralization
Development of USP <1227>
Method Suitability for Quantitative Studies
Other considerations in preservation
Container/Closure
In-use Testing
Investigations
Lab Investigations
Specific Considerations for AET
Summary/Conclusions
References
Monitoring of Microbiological Quality Attributes of Water for Pharmaceutical Use
by Dilip Ashtekar
Introduction
Water Types
Regulatory Requirements
Water System Description
Prefiltration
Activated Carbon
Softeners
Deionization
Reverse Osmosis
Ultrafiltration
Ultraviolet Light
Ozone
Final Filtration
Distillation
Storage Tanks
Distribution Systems
Water System Validation
Installation Qualification
System Characterization Studies and Testing
Operational Qualification
Performance Qualification
Phase I PQ Testing
Phase II PQ Testing
Post-Validation Monitoring
Water System Monitoring
Sampling and Frequency of Monitoring
Sample Collection and Testing
Alert and Action Levels
Response to Confirmed Alert and Action Level Excursions
Water Data Trending Requirements
Conclusions
References
Investigation of Microbiological Data Deviations
by Mónica Lagomarsino
Introduction
Microbiological Data Deviations
Objectives of the Investigation
Acceptance Criteria
General Process for the Investigation
Laboratory Investigation
Full-Scale Investigation
A Case Study
Conclusion
References
Recommended Bibliography
Alternative Microbiological Methods and New Pharmaceutical Microbiology Curriculum
by Claudio D. Denoya
Introduction
A “Rapid” History of Microbiology
Today’s Microbiology: The Fundamentals of “Traditional” Microbiology
The New Microbiological Technology Wave for the QC Lab: Alternative and Rapid
Microbiological Method (ARMMs)
Metabolic or Growth Based Technologies
Viability Based Technologies
Technologies Based on Cell Component Analysis
ARMMs and the Pharmaceutical Industry
AMM, PAT, and Regulatory Status
Microbiology Curricula
The Skill Sets of a Pharmaceutical Quality Control (QC) Microbiologist
Curricula Surveys
The Disparity between the Tertiary Microbiology Curricula and the Needs of the
Pharmaceutical Industry QC Microbiologist
An Interesting Step Forward: The United States Professional Science Master''''''''s
Programs
Conclusions and Recommendations
Acknowledgments
References
The Implementation of Rapid Microbiological Methods
by Michael J. Miller
Microbiology Trapped In the 19th Century
New Technologies for Pharmaceutical Manufacturing
An Introduction to Rapid Microbiological Methods
RMM Applications
Strategy for Implementation
Validating Rapid Microbiological Methods
Initial Activities
The Validation Strategy
Risk Assessment
Validation Master Plan (VMP)
User Requirements Specifications (URS)
Design Qualification (DQ)
Supplier Assessment/Audit
Functional Design Specifications (FDS)
Requirements Traceability Matrix (RTM)
Training and SOPs
The Test Plan
Installation Qualification (IQ)
Operational Qualification (OQ)
Validation Criteria for Quantitative Tests
Validation Criteria for Qualitative Tests
Validation Criteria for Microbial Identification Tests
Performance Qualification (PQ)
Validation Summary Report
Implementation and Secondary Site Qualification
RMMS and the Regulatory Environment
FDA Perspectives
Pharmaceutical cGMP’s for the 21st Century: A Risk-Based Approach
Process Analytical Technology (PAT)
Sterile Drug Products Produced by Aseptic Processing – cGMP
RMM’s for Sterility Testing of Cellular and Gene Therapy Products
Encouragement from FDA’s Microbiology Review and Compliance Staff
Strategies for Implementing RMMs with the FDA
EMA Perspectives
EMA Revised Variations Regulation
PDA Forum on Implementing RMMs in Europe
Type Variations
Scientific Advice
RMM Validation
The EMA PAT Team
Changing Acceptance Levels and Specifications
Regulatory Summary
Developing a Business Case for RMMS
Where Can Savings Come From?
Creating an Economic Analysis
Operating Costs Associated with the Conventional Method
Operating and Investment Costs Associated with the RMM
Cost Savings/Cost Avoidances Associated with the RMM
Return on Investment (ROI)
Payback Period (PP)
Net Present Value (NPV)
Chapter Summary
Acknowledgement
References
Risk Management in Pharmaceutical Microbiology
by Tim Sandle
Introduction to Risk Assessment and Risk Management Process
Regulatory Views and Guidelines on Risk Management
The Basics of Risk Assessment
Advantages and Disadvantages of Risk Assessment
Risk Assessment and Risk Management Methodologies
HACCP: Risk Based Approach in Environmental Monitoring
Case Study 1
Deconstructing the Process
Route Map
Identification of Hazards
Process Flow
Environmental Monitoring
Risk Assessment
Perform a Simulation
Evaluation
FMEA - Risk Based Approach in Sterility Testing
Case Study 2
Sterility Testing Isolator: The Case Study
Description of the System
Application
The FMEA Study on the Sterility Testing Isolator System
Designing the FMEA Scheme
The FMEA Exercise
Examination 1: The Isolator Room
Examination 2: Potential of Sanitisation Cycle Failure
Examination 3: Frequency of Isolator Sanitisations
Examination 4: Compromise of Isolator Integrity
Examination 5: Connection of Transfer Isolator to Main Isolator and
Transfer-in / out of Material
Examination 6: Incomplete Transfer Isolator Sanitisation
Examination 7: Failure of a Daily, Weekly or Six-monthly Physical
Parameter - HEPA filters / Pressure Leaks to Canopy
Examination 8: Pressure Leaks to Gloves
Summary
Other Available Tools
Ishikawa or Fishbone Diagrams
Risk Ranking
Risk Filtering
Risk Modelling
Contradiction Tables or Matrix
Conclusion: The benefits of Risk management
References
Part: 4 Sterilization and Sterility Assurance
Sterility
by Edward C. Tidswell
Introduction
Origins of Sterilization
The Microbial Challenge
Consequences of Non-Sterility and Lack of Asepsis
What Does Sterile Mean and Can We Test It?
Conclusion
References
Process Selection for Sterile Products
by James Agalloco
Introduction
Terminal Sterilization and Adjunct Processing
Aseptic Processing
Conclusion
References
Microbial Contamination Control in Pharmaceutical Manufacturing
by Matts Ramstorp
Pharmaceutical Cleanrooms and Clean Zones
Introduction
The Use of Proper Hygiene
The Historic Development of Microbiology
Aseptic Techniques
Production of Medicinal Products
Why is Pharmaceutical Production Different from Microelectronics?
Classification of Pharmaceutical Cleanrooms
Good Manufacturing Practice (GMP)
Design of Pharmaceutical Cleanrooms
How to Proceed with the Design of a Pharmaceutical Cleanroom
Cleanroom Cleanliness
Pharmaceutical Cleanroom Classification
Measuring Cleanliness within Pharmaceutical Cleanrooms
Particle Counting
Microbial Monitoring
Analysis of Microbiological Contaminants in the Air
Sampling of Microorganisms
Sampling of Microorganisms in the Air of a Cleanroom
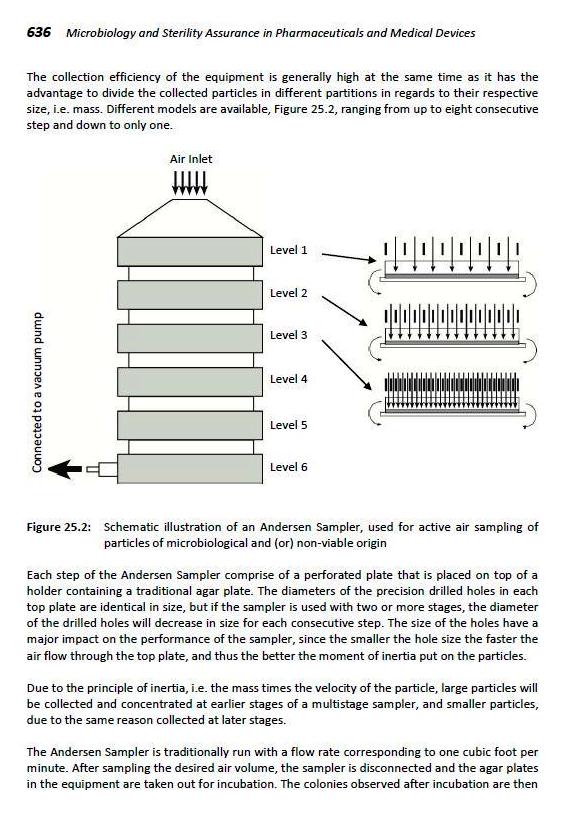
The Andersen Sampler
Slit-to-Agar-Sampler
The RCS-Sampler
Liquid Impinger
Surface Sampling
Microbiological Contaminants and GMP
Pharmaceutical Cleanroom Ventilations Systems
Conventional Ventilated Cleanrooms
Unidirectional Flow Cleanrooms
Ventilation Filter for Pharmaceutical Cleanrooms
Cleanrooms and Clean Zones
Working in Clean Zones
Pharmaceutical Cleanroom Production Systems
Production using Terminal Sterilization
Aseptic Production
Cleaning and Disinfection of a Pharmaceutical Cleanroom
Introduction
What is the Purpose of Cleaning?
What is Cleaning?
How to Work with Preventive Cleaning
Active Cleaning
Classification of Surfaces in a Cleanroom
Cleaning Program for Cleanrooms
Example of a Cleaning Program
How to Analyse and Control Cleaning
Cleaning Techniques
Cleaning Methods
Dry Cleaning Methods
Wet Cleaning Methods
Cleaning Solution
The Zinner Circle
Disinfection
Potential Risk Factors Associated with Cleanroom Decontamination
Standards and Practices
Cleanroom Garments
Introduction
What is the Purpose of the Cleanroom Garment?
Garment Systems
Single-use versus Washable Garments
Choice of Material
Comfort
Construction of Garment Systems
The EU GMP and Cleanroom Garment Systems
Using Cleanroom Garments
Clothing, Personal Items and Underwear
Processing of Clothing and Change Frequency
Risk Factors Associated With Cleanroom Clothing
Conclusions
Personal Hygiene and Personal Responsibility
Introduction
Humans as Particle Generators
Particles and Fibres
The Normal Flora of Man
Good Manufacturing Practice in Relation to Personnel
Personal Hygiene
The Interconnection between Personnel and the Process
Contamination Hazards Connected to Personnel
General Rules when Working in Cleanrooms and other Controlled Environments
Acknowledgement
References
Aseptic Process Simulations/Media Fills
by Marco Budini and Francesco Boschi
Introduction
Documentation
Media Selection
Frequency and Number of Runs
Size and Duration of Runs
Line Speed and Container Size
Closure Type
Process/Line Configuration and Set-up
Fill Volume
Number of Persons and Activities
Interventions
Line Clearance
Environmental and Personnel Monitoring
Other Worst Case Scenarios
Cleaning after Process Simulation
Incubation and Inspection of Filled Units
Growth Promotion Test
Interpretation of Results – Acceptance Criteria
Invalidation and Abortion of Process Simulation Runs
Investigation of Process Simulation Contaminations/Failures
Conclusions
References
Biological Indicators for Sterilization
by Russ Nyberg
Description
Performance Qualification of Biological Indicators
BI Use in Parenteral Product Loads
Use of a Process Challenge Device (PCD)
Selection of BIs
Contracting for 3rd Party D-Value Testing
Equipment
Test Method Used
Recovery Media
Technique and Lab Utensils/Personnel
Rapid Read-out Biological Indicators
Conclusion
References
Moist Heat Sterilization
by Michael Sadowski
Introduction
Sterile Products
History of Sterilization with Heat
Mechanisms of Spore Heat Resistance And Inactivation
Inactivation of Spores Using Moist Heat Sterilization Processes
Use of Semilogarithmic Survivor Curve Model for Characterizing and Predicting Microbiological Inactivation
Pharmaceutical Products
Selection of a Moist Heat Sterilization Process Type
Saturated Steam Sterilization Processes
Gravity Displacement Processes
Pre-vacuum Processes
Air Overpressure Processes
Steam/Air Mixture Processes (SAM)
Superheated Water Air Overpressure Processes
Product or Item Loading Patterns
Biological Indicators for the Development and Qualification of Moist Heat Sterilization Processes
Determination of The Hardest to Sterilize Solution Formulation or Item/Component
The Liquid Products Master Solution Approach
The Process Challenge Device (PCD) Approach for Porous/Hard Goods
Determination of Hardest To Sterilize Locations or Cold Spots Used For Penetration Probes
Use of Chemical Indicators
Development Moist Heat Sterilization Processes
Development of the Sterilization Process Heat Up/Conditioning Phase
Development of the Sterilization Process Cooling/Drying Phase
Development of the Exposure Phase
Determination of the Minimum Physical Lethality Value
Minimum Physical Lethality Values Prescribed in Regulatory Standards
Minimum Physical Lethality Values Required for the Overkill Cycle Design Approach
Determination of Minimum Physical Lethality Values for the Product Specific Approach
Determination of Exposure Time to Meet Minimum Physical Lethality Requirements
Use of Biological Lethality in the Determination of Exposure Time
Use of the Fractional Exposure Approach in the Determination of Exposure Time
Validation of The Moist Heat Sterilization Process
Installation Qualification
Operational Qualification
Pre-vacuum Sterilizer Vacuum Leak Rate Test
Steam Penetration or Bowie Dick Type Test
Steam Quality Tests
Performance Qualification
Use of Physical Lethality in the Qualification of Moist Heat Sterilization Processes
Use of Biological Lethality in the Qualification of Moist Heat Sterilization Processes
Routine Monitoring and Control of the Moist Heat Sterilization Process
Preventive and Unplanned Maintenance
Calibration Program
Ongoing Sterilizer and Utility System Functionality Tests
Product and Process Change Control
Sterile Product Release Process
Bioburden Testing
Sterilizer Functionality
Sterilization Cycle Parameters
Chemical Indicator and Biological Indicator Results
The Sterility Test
Parametric Release
Conclusion
Acknowledgement
References
Sterilization and Depyrogenation by Dry Heat
by Madhu Raju Saghee and Gary R. Mitchel
Introduction
Functions of Dry Heat
Thermodynamical Aspects of Heat Transfer in Dry Heat Processes
Destruction of Microorganisms and Endotoxins by Dry Heat
Spectrum of Activity on Microbial Populations
Effect of Microbial Water Content in Dry Heat Sterilization Processes
Effect of Temperature and Time
Destruction of Endotoxins
Types of Dry Heat Sterilization/Depyrogenation Processes
Batch Process
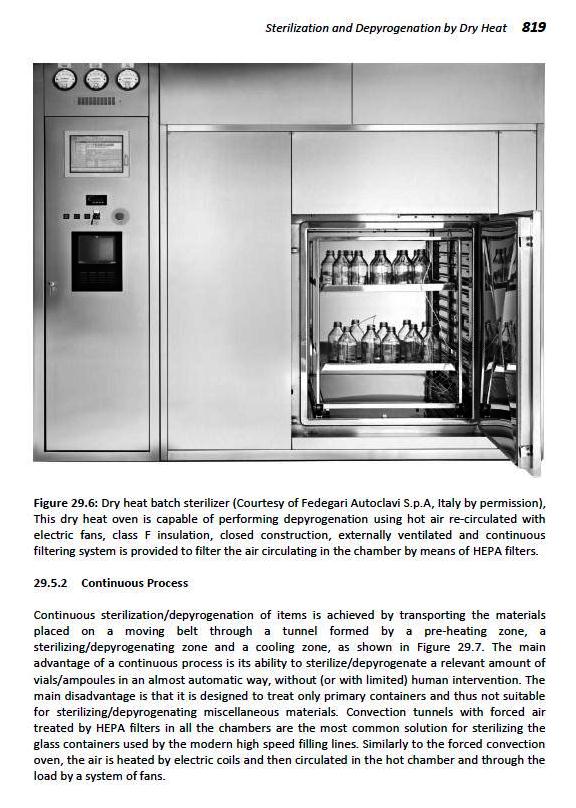
Continuous Process
Validation and Ongoing Control
Temperature Measurement Errors
Validation Approach
Installation Qualification
Operational Qualification
Performance Qualification
Routine Monitoring and Control
Conclusion
References
Acknowledgements
Appendix A (Example of an outline for an Installation Qualification protocol)
Appendix B (Example of an outline for Operational and Performance Qualification protocols)
Radiation Sterilization
by Mark A. Seybold and John A. Williams
Introduction
History of Radiation Sterilization
Types of Radiation Sterilization
Gamma Sterilization
Electron Beam Sterilization
X-Ray Sterilization
Radiation Sterilization Applications
Microbial Inactivation
Mode of Action
Microbial Resistance to Ionizing Radiation
Radiation Resistance Factors
Environmental Factors
Organism Characteristics
Inactivation Kinetics
Validation
Installation Qualification
Operational Qualification
Performance Qualification
Microbiological Validation of Radiation Sterilization Processes
Dose Setting
Method 1
Method 2
Method VDmax
Materials Qualification
Process Validation
Routine Monitoring And Control
Maintaining Process Effectiveness
Biorburden Monitoring Program
Dose Audits
Radiation Equipment
Conclusions
References
Sterilization by Filtration
by Maik W. Jornitz and Theodore H. Meltzer
Introduction to Sterilizing Filtration
Filtration Parameters
Contamination Removal
Rate of Flow
Total Throughput
Unspecific Adsorption
Filter Types
Filter Materials
Filter Construction
Filter Validation
Filter Integrity Testing
Bubble Point Test
Diffusion Test
Pressure Hold Test
Water Intrusion Test
Product Wet Integrity Testing
Filtration Applications
Liquids
Solvent (API) Filtration
Ophthalmics Filtration
Cell Culture Media
Buffer Filtration
Gases
Fermentor Inlet Air
Fermentor Off-Gas
Vent Filters on Tanks
Autoclave and Lyophilizer Vent Filter
Filtration of Service Gases
References
Sterilization by Ethylene Oxide
by Gerry A. O’Dell
Introduction
Factors Affecting the Lethality of EO Sterilization
Gas Concentration
Humidity/Moisture
Temperature
Inherent resistance of microorganisms
Conferred resistance
Combination of factors
The EO Sterilization Process
Preconditioning
Sterilization Cycle
Aeration
Product and Process Definition for EO Sterilization
Product definition
Process definition
EO Sterilization Validation
Installation Qualification
Operational Qualification
Performance Qualification
Microbiological PQ
Physical PQ
Routine monitoring of the EO sterilization process
Conventional Release
Parametric Release
Use of tests for sterility
Demonstrating the Ongoing Effectiveness of the EO Sterilization Process
Addressing changes (change control)
Process equivalence
Product adoption
Periodic requalification
Summary
References
Maintaining Sterility
by Michelle A. Luebke & Bonnie J. Heredia
Introduction to Maintaining Sterility
Medical Product Packaging System Development
Packaging Development Phase
Routine Manufacturing/In-Process Control Phase
Stability/Product Testing Phase
Microbiological Versus Physical Integrity Test Methods
Test Method Correlation
Direct Approach
Indirect Approach
Test Method Sensitivity
Test Method Validation
Method and Vendor Requirements
Validation Plan
Installation Qualification
Operational Qualification
Performance Qualification
Sterile Medical Product Classification
Medical Devices
Medical Devices - Sterile Designation
Medical Devices - Sterile Fluid Path Device Designation
Needleless Medical Devices
Drugs and Biologics
Sterile Barrier System Classification
Seals
Closures
Sterile Barrier Integrity Test Methods
Physical Methods
Visual Inspection
Bubble Test
Pressure/Vacuum Decay
Dye Test
Tracer Gas Leak Detection
Microbiological Methods
Microbial Challenge - Liquid Immersion
Microbial Challenge - Aerosolization
Static Aerosol Challenge
Dynamic Aerosol Challenge
Simulated Clinical Use Test Method
Miscellaneous Methods
Summary
References
Index