Content
CONTENTS
1 Fundamentals of Global GMP Requirements
by Atul Shirgaonkar
Introduction
What are GMPs
History and Evolution of GMPs
Major International GMPs
Quality Management
Product Quality Review
Quality Risk Management
GMP: Differences between US FDA and EU
Requirements
Conclusion
References
2 Effective CAPA Management for Optimal
Compliance
by Michael Hopper
Introduction
Examples of CAPA Issues
Global 8D
Human Error
Approaches to CAPA
Summary
References
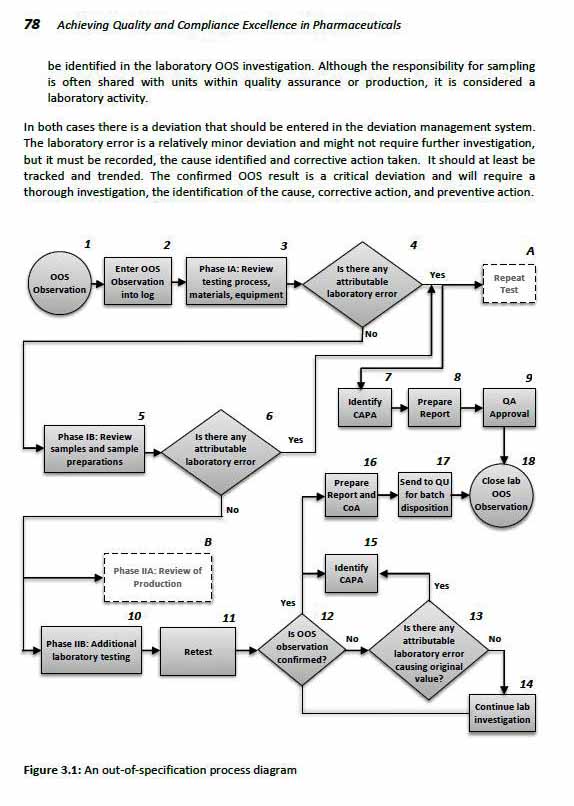
3 Laboratory Compliance and Handling Out-of-
Specification (OOS) Results in the Laboratory
by John Lanese and A.V. Prabhu
Laboratory Controls
Handling Out of Specification (OOS) Results
Phase I of the OOS Investigation
Phase II of the OOS Investigation
Conclusion
References
4 Effectively Incorporating Quality Risk
Management into Quality Systems
by Tim Sandle and Sanjit Singh Lamba
Introduction
Quality Systems
Guidelines
Anatomy of ICH Q9
Applying Quality Risk Management
The Basics of Risk Assessment
Risk Assessment Tools
The Integration of QRM into Some Process Quality
Systems
Advantages and Disadvantages of Risk Assessment
Conclusion
References
5 Monitoring and Controlling Process Drift for
Enhancing Quality
by Nandkumar Chodankar
Introduction
Definition of Process Drift
Chance Cause
Background of Process Development and Process
Validation
Process Variables (Identified Chance Cause)
Combined Effect of Variables
What Could be the Additional Causes for Process
Drift
Categorizing Process Drift
Approaches to Developing a Control Strategy
Steady State Concept
Development of Critical Strategy for Process
Modelling Control Design
PAT: A Tool for Online Process Control
Steps involved in API Manufacturing and Their
Control
Steps involved in Drug Product Manufacturing and
Their Control
Summary
References
6 Qualification and Validation
by Tim Sandle
Introduction
Regulations
History of Validation
Validations Concepts and Documentation
Risk Based Approaches to Validation
Re-Validation Requirements
Different Types of Validation: Case Studies
Conclusion
References
7 Process Validation
by Mark F. Witcher
Introduction
History of Manufacturing Controls & Process
Validation
Design Space - Defining and Describing the
Manufacturing Process
FDA''s 2011 Validation Paradigm
Product Lifecycle
The Role of QbD in Process Validation
Understanding the Process Validation Paradigm
Using the Process Validation Paradigm
Quality Risk Management (QRM) for Process
Validation
Legacy Products
Validation Master Plan (VMP)
References
8 Documents, Records, and Part 11 Compliance
by Janet Gough
Introduction
Documentation and Regulation
The Document Continuum
Features of Robust Systems
Part 11
Deciding to go Electronic
System Documentation
Maintaining Compliance and Inspection Readiness
References
9 Change Control and Management
by R. Raghunandanan
Introduction
Key Definitions
Categorization of Different Changes
Why Change Control
Regulatory Requirements
GMP Requirements
Business Requirements
Change Control Management Process
Documentation
Example of FDA Warning Letter
References
10 Deviation Management
by Alicia Tébar Pérez
Introduction
Deviations - Regulatory Framework
What Constitutes a Deviation
Deviation Management Flowchart
Deviations into the Quality System
Common Deficiencies Related to Poor Deviation
Management
Deviation Management from a Risk/Science Based
Perspective
Applying Risk Analysis to Deviation Management
Deviations and Knowledge Management for
Continuous Improvement
Advanced Tools for Deviation Management
and Reduction
Conclusions
Acknowledgements
References
11 Internal Quality Assessments/Self-Audits
by R. Raghunandanan
Introduction
Key Definitions
Why Internal Quality Audits
Regulatory Requirements
Who Carries Out Audits
Auditor Requisites
Auditing Process
Reporting and CAPA
Why Audits Fail
Need to Strengthen Internal Audit System
12 Designing an Effective GMP Training Program
by David Markovitz
Introduction
GMP Training is a Process
GMP Training - A Process to Ensure Compliance
Proper Preparation for Effective GMP Training
Conducting Effective GMP Training: Dos and Donts
for Success
Effective Followup Strategiesto GMP Training
Start with the Basics
Conclusion
13 Behavioural GMPS (bGxP®): A New Paradigm
in Compliance Management
by Brian Szukala
Introduction: Challenges Facing the Industry
Surviving in a World of ''Non-Compliance''
Origins of bGxP
The Concept of Behavioural Compliance
Drivers for Behavioural Change - PIMS Model
First Step in the Process - The ICE Audit
Human Error and bbCi
Sustaining the Change and Future Developments
in GxP
References
14 Supplier Quality Management
by Ajit Basrur and David Stephon
Introduction
Government Regulatory Drivers
Applicable Regulations/Guidelines
Supplier Controls
Sub Tier Suppliers
References
Key Terms
15 Understanding the United States Pharmacopeia
(USP)
by Robert D. Seltzer
Introduction
General Notices
Mandatory USP General Chapter Key Takeaways
Informative USP General Chapter Key Takeaways
Acknowledgements
References
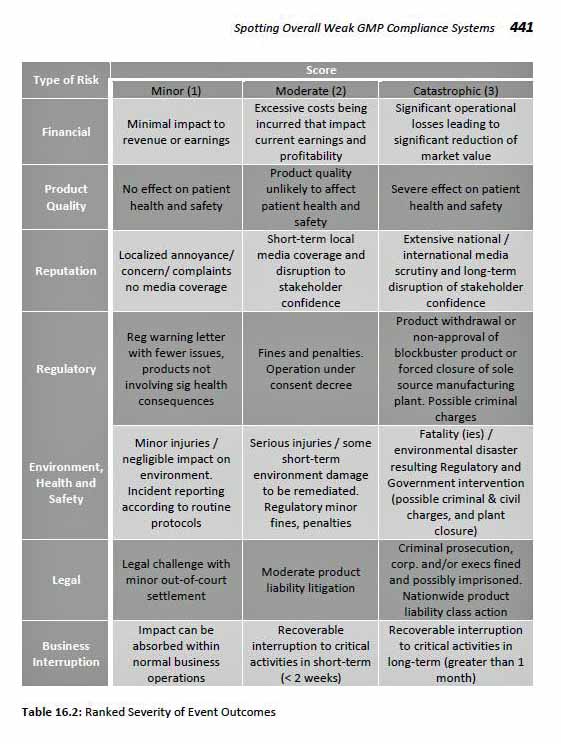
16 Spotting Overall Weak GMP Compliance
Systems
by Robert D. Seltzer
Introduction
Key Definitions, Fallacies in Logic and GMP
Literacy
Risk Categorized and Semi-Quantified
Trigger Events for Investigation, Remediation and
Corrective Action
Causes and Effects
Product Quality Complaints
Corrective Action Completeness and Effectiveness
Concluding Remarks and Examples
Acknowledgements
References
17 Meaningful Performance Metrics for
Compliance
by Roger Janczak
Overview of Performance Metrics
Principles of Establishing Performance Metrics for
Compliance
Compliance Metrics
Reviews, Scorecards and Dashboards
Other Considerations
References
18 Implementing ICH Q 10: A Pragmatic Approach
by Alok Ghosh and Nilanjana Basu
Introduction: What is ICH Q10
Key Messages of ICH Q10
Roles and Responsibilities of Senior Management
Product Lifecycle Covered by PQS
Continual Improvement
Enablers of PQS
Manufacturing Implementation & PQS
Conclusive Remarks
Acknowledgement
References
19 Compliance Aspects of APIs Manufacturing
by Richard Einig
Introduction
Quality Management and Personnel
Facilities, Equipment and Utilities
Production
Laboratory Control and Stability Studies
Conclusion
References
20 Compliance Aspects of Sterile Manufacturing
by Tim Sandle and Madhu Raju Saghee
Introduction
Sterility
Methods of Producing Sterile Products
A Brief History of Compliance Pertaining to Sterile
Products
Compliance Aspects of Sterile Manufacturing
Important Compliance Issues
Summary
References
21 Domestic and International U.S. Food and Drug
Administration (FDA) Inspections
by Robert D. Seltzer
Introduction
Types of Inspections
Inspections of Foreign Drug Manufacturers
Inspection Techniques
FDA Inspection Preparedness and Handling
Implications from FDA''s Admission to the PIC
Acknowledgements
References
22 Avoiding FDA Enforcement Actions: An
Optimal & Sustainable Compliance Program
by Areta Kupchyk
Introduction
FDAs Legal Authority
FDA Enforcement
Post Approval Requirements, Compliance,
and Enforcement
Other FDA Compliance and Enforcement Methods
Tools
Concusions and Recommendations
23 Developing a Master QMS Plan
by John E. Lincoln
Introduction
Development of "Quality" in the 21st Century
Master QMS Plan - Product Use Risk Based
Developing a Master QMS Plan
Quality Systems at US FDA and EU
Modern Quality Systems
A Pragmatic Framework for Building a Culture of
Compliance
Conclusion
24 Trends in cGMP Compliance
by Mitch Manning
Introduction
What are Trends in GMP Compliance
Why Identify GMP Compliance Trends
How to Identify and Use GMP Compliance Trends
Conclusion: Trends in GMP Compliance
References
Content
CONTENTS
1 Fundamentals of Global GMP Requirements
by Atul Shirgaonkar
Introduction
What are GMPs
History and Evolution of GMPs
Major International GMPs
Quality Management
Product Quality Review
Quality Risk Management
GMP: Differences between US FDA and EU Requirements
Conclusion
References
2 Effective CAPA Management for Optimal Compliance
by Michael Hopper
Introduction
Examples of CAPA Issues
Global 8D
Human Error
Approaches to CAPA
Summary
References
3 Laboratory Compliance and Handling Out-of-Specification (OOS) Results in the Laboratory
by John Lanese and A.V. Prabhu
Laboratory Controls
Handling Out of Specification (OOS) Results
Phase I of the OOS Investigation
Phase II of the OOS Investigation
Conclusion
References
4 Effectively Incorporating Quality Risk Management into Quality Systems
by Tim Sandle and Sanjit Singh Lamba
Introduction
Quality Systems
Guidelines
Anatomy of ICH Q9
Applying Quality Risk Management
The Basics of Risk Assessment
Risk Assessment Tools
The Integration of QRM into Some Process Quality Systems
Advantages and Disadvantages of Risk Assessment
Conclusion
References
5 Monitoring and Controlling Process Drift for Enhancing Quality
by Nandkumar Chodankar
Introduction
Definition of Process Drift
Chance Cause
Background of Process Development and Process Validation
Process Variables (Identified Chance Cause)
Combined Effect of Variables
What Could be the Additional Causes for Process Drift
Categorizing Process Drift
Approaches to Developing a Control Strategy
Steady State Concept
Development of Critical Strategy for Process Modelling Control Design
PAT: A Tool for Online Process Control
Steps involved in API Manufacturing and Their Control
Steps involved in Drug Product Manufacturing and Their Control
Summary
References
6 Qualification and Validation
by Tim Sandle
Introduction
Regulations
History of Validation
Validations Concepts and Documentation
Risk Based Approaches to Validation
Re-Validation Requirements
Different Types of Validation: Case Studies
Conclusion
References
7 Process Validation
by Mark F. Witcher
Introduction
History of Manufacturing Controls & Process Validation
Design Space - Defining and Describing the Manufacturing Process
FDA's 2011 Validation Paradigm
Product Lifecycle
The Role of QbD in Process Validation
Understanding the Process Validation Paradigm
Using the Process Validation Paradigm
Quality Risk Management (QRM) for Process Validation
Legacy Products
Validation Master Plan (VMP)
References
8 Documents, Records, and Part 11 Compliance
by Janet Gough
Introduction
Documentation and Regulation
The Document Continuum
Features of Robust Systems
Part 11
Deciding to go Electronic
System Documentation
Maintaining Compliance and Inspection Readiness
References
9 Change Control and Management
by R. Raghunandanan
Introduction
Key Definitions
Categorization of Different Changes
Why Change Control
Regulatory Requirements
GMP Requirements
Business Requirements
Change Control Management Process
Documentation
Example of FDA Warning Letter
References
10 Deviation Management
by Alicia Tébar Pérez
Introduction
Deviations - Regulatory Framework
What Constitutes a Deviation
Deviation Management Flowchart
Deviations into the Quality System
Common Deficiencies Related to Poor Deviation Management
Deviation Management from a Risk/Science Based Perspective
Applying Risk Analysis to Deviation Management
Deviations and Knowledge Management for Continuous Improvement
Advanced Tools for Deviation Management and Reduction
Conclusions
Acknowledgements
References
11 Internal Quality Assessments/Self-Audits
by R. Raghunandanan
Introduction
Key Definitions
Why Internal Quality Audits
Regulatory Requirements
Who Carries Out Audits
Auditor Requisites
Auditing Process
Reporting and CAPA
Why Audits Fail
Need to Strengthen Internal Audit System
12 Designing an Effective GMP Training Program
by David Markovitz
Introduction
GMP Training is a Process
GMP Training - A Process to Ensure Compliance
Proper Preparation for Effective GMP Training
Conducting Effective GMP Training: Dos and Donts for Success
Effective Followup Strategiesto GMP Training
Start with the Basics
Conclusion
13 Behavioural GMPS (bGxP®): A New Paradigm in Compliance Management
by Brian Szukala
Introduction: Challenges Facing the Industry
Surviving in a World of ''Non-Compliance''
Origins of bGxP
The Concept of Behavioural Compliance
Drivers for Behavioural Change - PIMS Model
First Step in the Process - The ICE Audit
Human Error and bbCi
Sustaining the Change and Future Developments in GxP
References
14 Supplier Quality Management
by Ajit Basrur and David Stephon
Introduction
Government Regulatory Drivers
Applicable Regulations/Guidelines
Supplier Controls
Sub Tier Suppliers
References
Key Terms
15 Understanding the United States Pharmacopeia (USP)
by Robert D. Seltzer
Introduction
General Notices
Mandatory USP General Chapter Key Takeaways
Informative USP General Chapter Key Takeaways
Acknowledgements
References
16 Spotting Overall Weak GMP Compliance Systems
by Robert D. Seltzer
Introduction
Key Definitions, Fallacies in Logic and GMP Literacy
Risk Categorized and Semi-Quantified
Trigger Events for Investigation, Remediation and Corrective Action
Causes and Effects
Product Quality Complaints
Corrective Action Completeness and Effectiveness
Concluding Remarks and Examples
Acknowledgements
References
17 Meaningful Performance Metrics for Compliance
by Roger Janczak
Overview of Performance Metrics
Principles of Establishing Performance Metrics for Compliance
Compliance Metrics
Reviews, Scorecards and Dashboards
Other Considerations
References
18 Implementing ICH Q 10: A Pragmatic Approach
by Alok Ghosh and Nilanjana Basu
Introduction: What is ICH Q10
Key Messages of ICH Q10
Roles and Responsibilities of Senior Management
Product Lifecycle Covered by PQS
Continual Improvement
Enablers of PQS
Manufacturing Implementation & PQS
Conclusive Remarks
Acknowledgement
References
19 Compliance Aspects of APIs Manufacturing
by Richard Einig
Introduction
Quality Management and Personnel
Facilities, Equipment and Utilities
Production
Laboratory Control and Stability Studies
Conclusion
References
20 Compliance Aspects of Sterile Manufacturing
by Tim Sandle and Madhu Raju Saghee
Introduction
Sterility
Methods of Producing Sterile Products
A Brief History of Compliance Pertaining to Sterile Products
Compliance Aspects of Sterile Manufacturing
Important Compliance Issues
Summary
References
21 Domestic and International U.S. Food and Drug Administration (FDA) Inspections
by Robert D. Seltzer
Introduction
Types of Inspections
Inspections of Foreign Drug Manufacturers
Inspection Techniques
FDA Inspection Preparedness and Handling
Implications from FDA''s Admission to the PIC
Acknowledgements
References
22 Avoiding FDA Enforcement Actions: An Optimal & Sustainable Compliance Program
by Areta Kupchyk
Introduction
FDAs Legal Authority
FDA Enforcement
Post Approval Requirements, Compliance, and Enforcement
Other FDA Compliance and Enforcement Methods Tools
Concusions and Recommendations
23 Developing a Master QMS Plan
by John E. Lincoln
Introduction
Development of "Quality" in the 21st Century
Master QMS Plan - Product Use Risk Based
Developing a Master QMS Plan
Quality Systems at US FDA and EU
Modern Quality Systems
A Pragmatic Framework for Building a Culture of Compliance
Conclusion
24 Trends in cGMP Compliance
by Mitch Manning
Introduction
What are Trends in GMP Compliance
Why Identify GMP Compliance Trends
How to Identify and Use GMP Compliance Trends
Conclusion: Trends in GMP Compliance
References